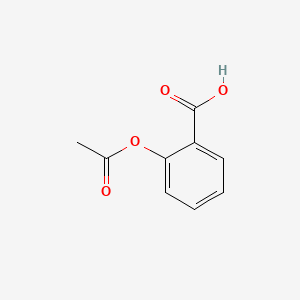
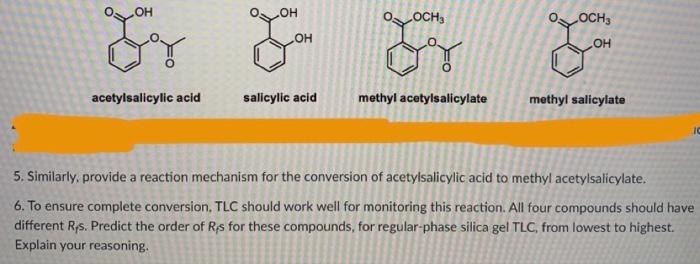
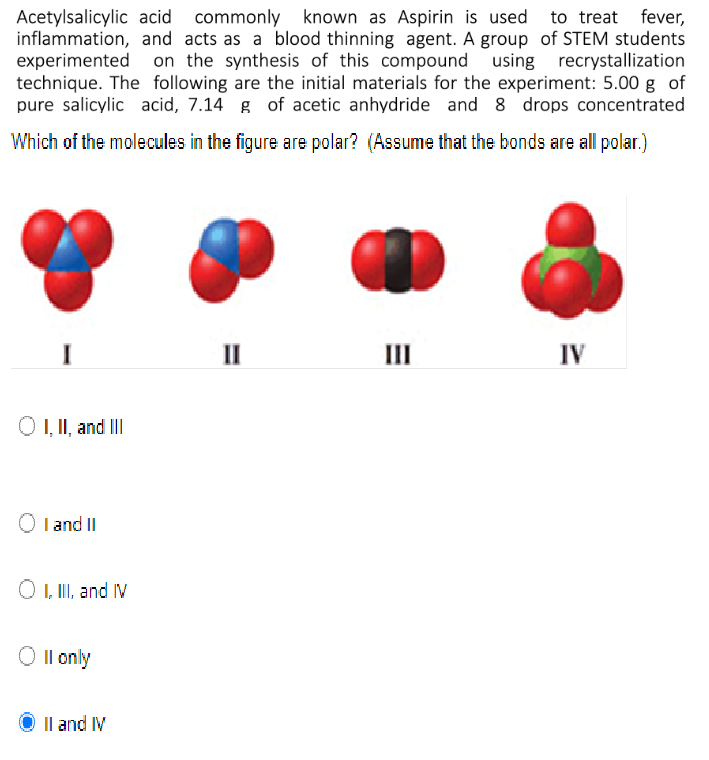
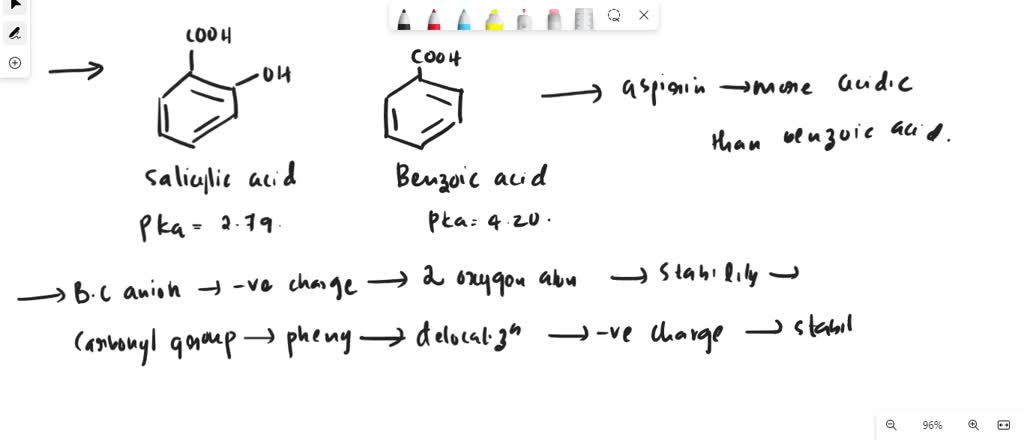
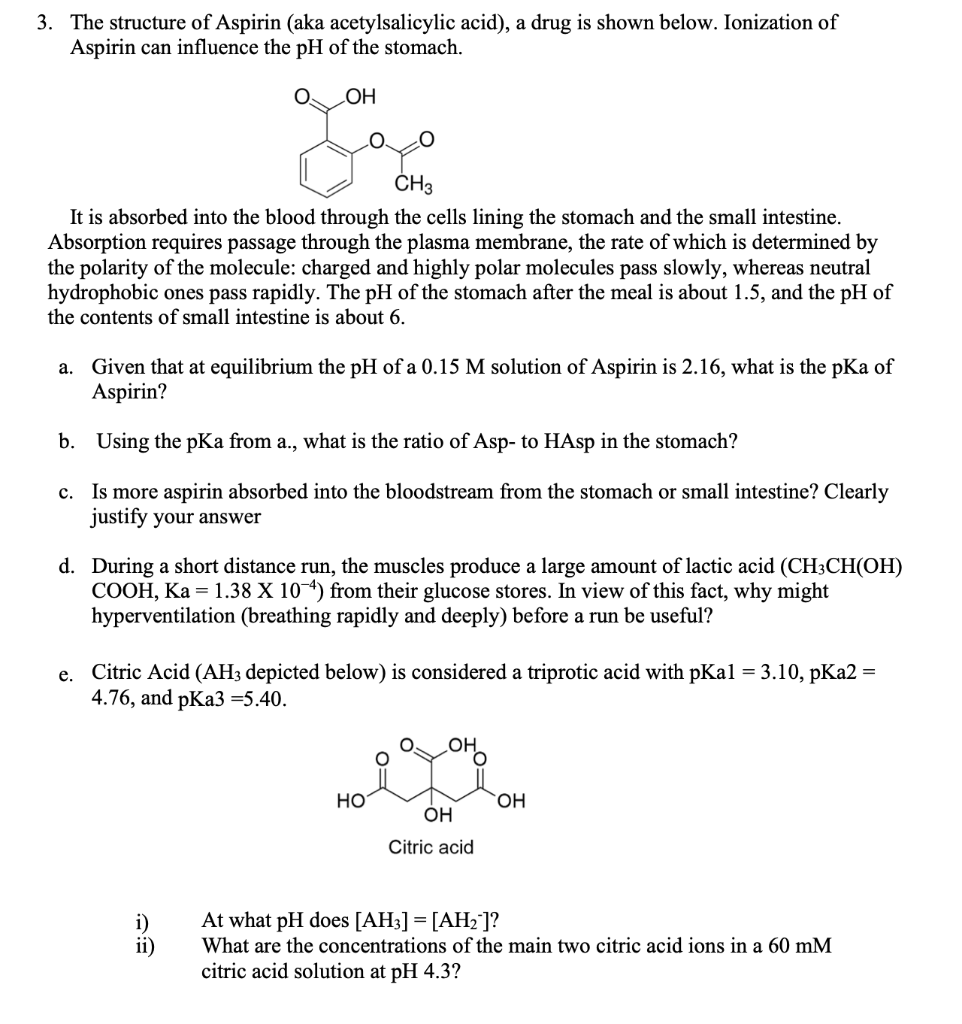
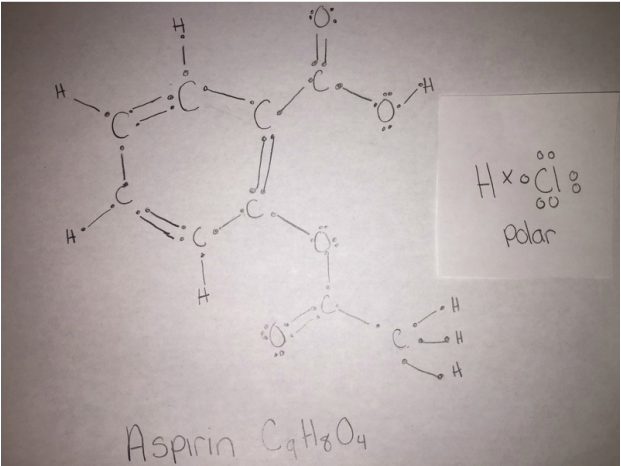
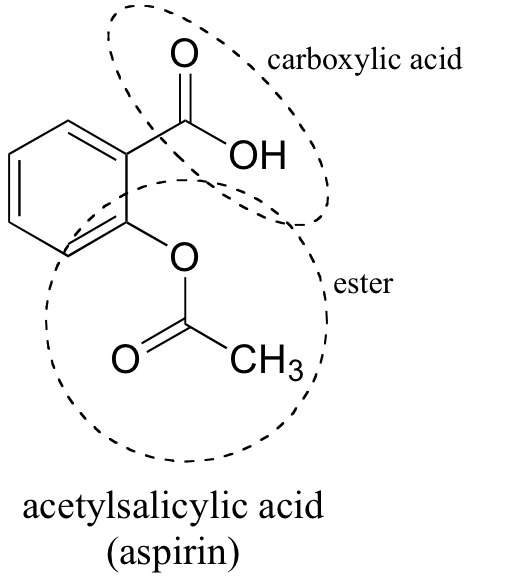
Following is a molecular model of aspirin (acetylsalicylic acid). Identify the hybridization of the orbitals on each carbon atom in aspirin, and tell which atoms have lone pairs of electrons (gray =

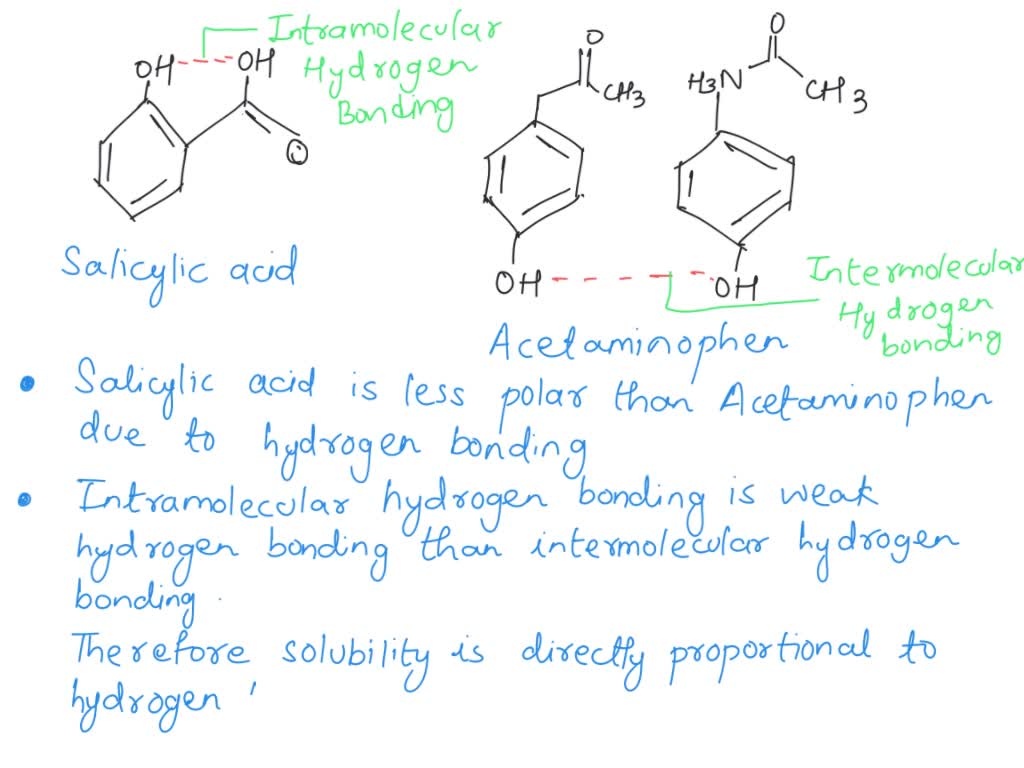
Which compound do you expect to be most polar: aspirin, acetaminophen, or caffeine? Explain. | Homework.Study.com

Salicylic acid, acetylsalicylic acid, methyl salicylate, salicylamide, and sodium salicylate in supercritical carbon dioxide: Solute – cosolvent hydrogen bonds formation - ScienceDirect